-
Ivan Buntic authoredIvan Buntic authored
- SFB Project Area C: Biomineralization
- What is Induced Calcite Precipitation (ICP)?
- What is ICP?
- Results of ICP
- Why investigate ICP?
- Why investigate ICP?
- Model concept
- Model concept: Relevant processes
- Model concept: Relevant processes
- Model concept: Relevant processes
- Model concept: Relevant processes
- MICP: Main reactions
- Model concept: Relevant processes
- Model concept: Relevant processes
- Equations
- Balance Equations
- Overall procedure of implementing chemical reactions in DuMu^\mathsf{x}:
- Sources & Sinks:
- Sources & Sinks:
- Sources & Sinks:
- Supplementary Equation:
- Specific Implementations
- Specific Implementations
- Biomineralization exercise
- Exercise
- Exercise tasks
- Exercise
title: Modelling porous medium modification </br><small>Induced Calcite Precipitation</small>SFB Project Area C: Biomineralization
What is Induced Calcite Precipitation (ICP)?
Microbes change the chemistry in a way that promotes the precipitation of calcite.
What is ICP?
 Credit: James Connolly, Montana State University.
Credit: James Connolly, Montana State University.
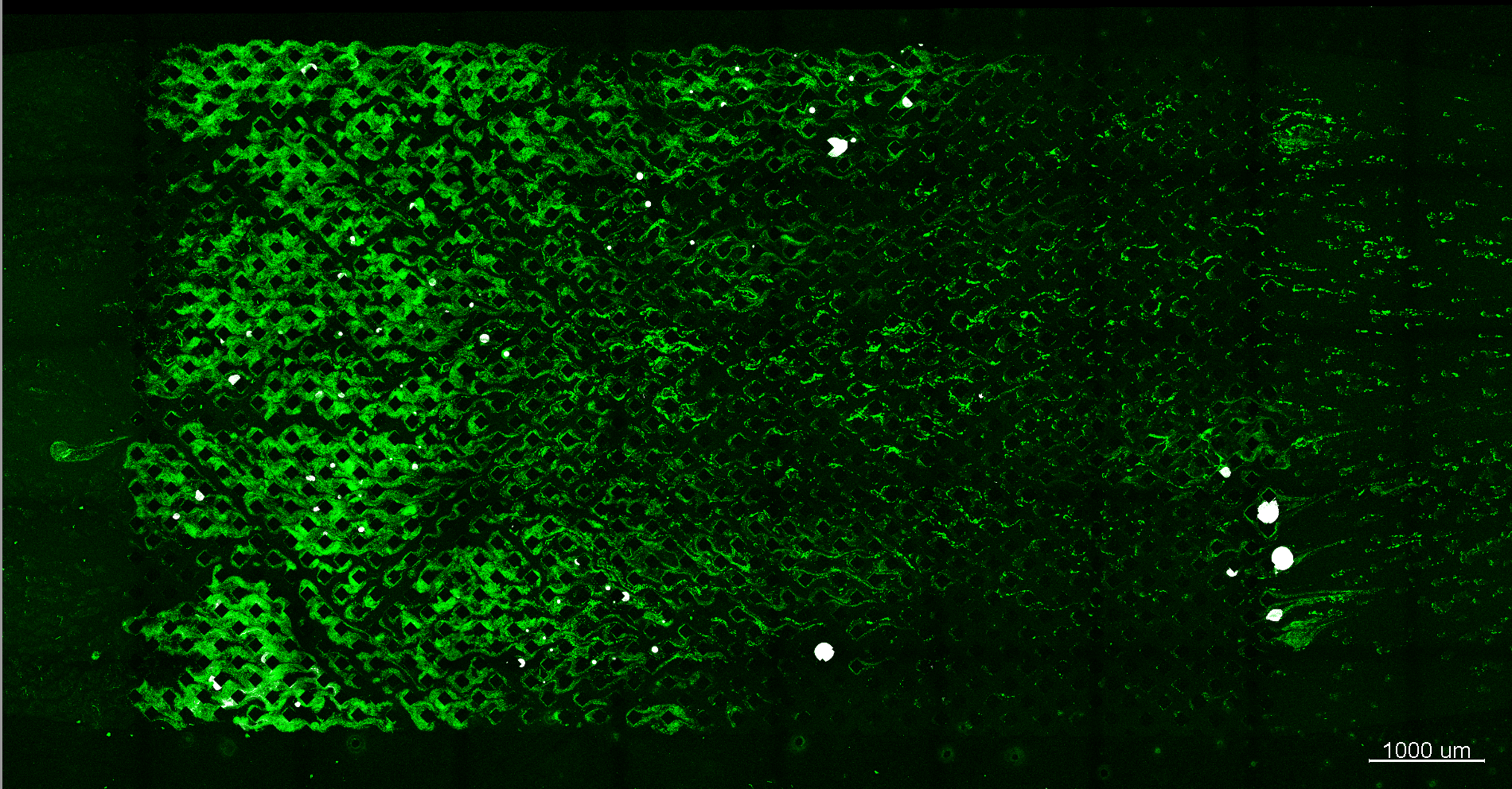
Results of ICP
Segmented CT image of a glass-bead column mineralized by ICP
:::::: {.columns}
::: {.column width=40%}
 :::
::: {.column width=60%}
:::
::: {.column width=60%}
 :::
::::::
Credit: Johannes Hommel, University of Stuttgart
:::
::::::
Credit: Johannes Hommel, University of Stuttgart
Why investigate ICP?
::::: {.columns}
::: {.column width=50%}
 Mineralized sand, photo by Johannes Hommel, University of Stuttgart.
:::
::: {.column width=50%}
Mineralized sand, photo by Johannes Hommel, University of Stuttgart.
:::
::: {.column width=50%}
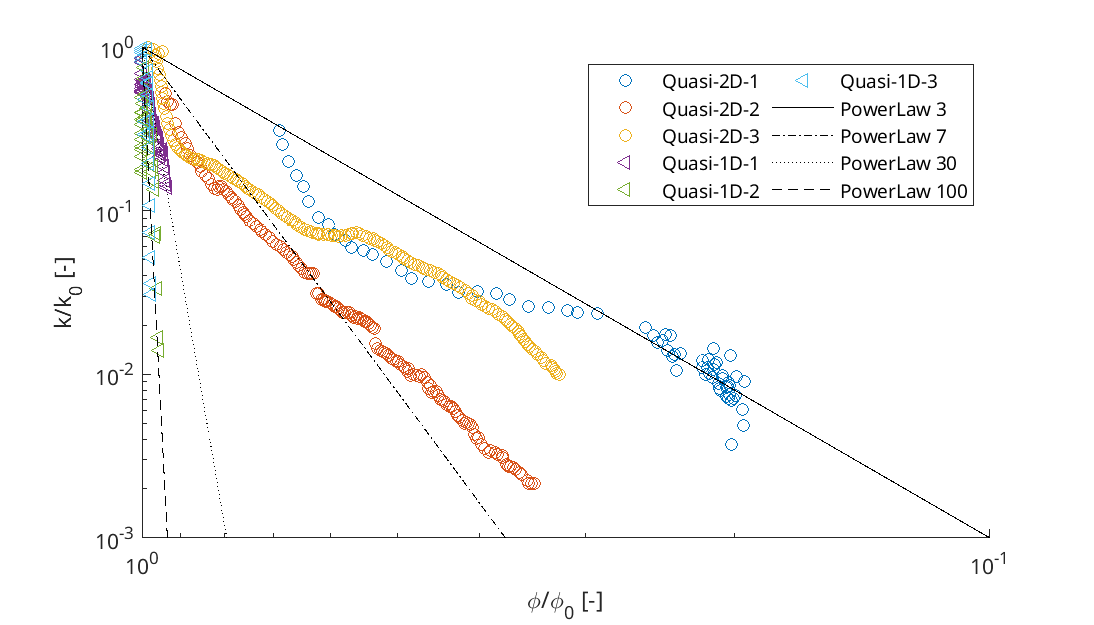 Porosity-Permeability changes observed by Felix Weinhardt, University of Stuttgart.
:::
::::::
Porosity-Permeability changes observed by Felix Weinhardt, University of Stuttgart.
:::
::::::
Why investigate ICP?
Applications in which a porous medium should be cemented in-situ, e.g. sealing, leakage mitigation, creating subsurface barries, reducing erosion, or stabilizing soil.
Main desired effects of ICP in those applications are:
- reduce flow (reduce K and k_r, increase p_c)
- increase mechanical strength
Model concept
Model concept: Relevant processes
:::::: {.columns}
::: {.column width=45%}
 (modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
(modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
- Two-phase transport ::: ::::::
Model concept: Relevant processes
:::::: {.columns}
::: {.column width=45%}
 (modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
(modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
- Two-phase, multi-component transport ::: ::::::
Model concept: Relevant processes
:::::: {.columns}
::: {.column width=45%}
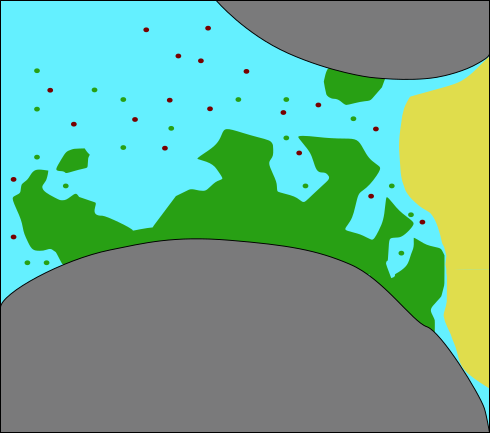 (modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
For this exercise:
Neglecting microbial growth and decay, attachment and detachment
(modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
For this exercise:
Neglecting microbial growth and decay, attachment and detachment
- Biomass (S. pasteurii)
- growth / decay
- attachment / detachment ::: ::::::
Model concept: Relevant processes
:::::: {.columns}
::: {.column width=45%}
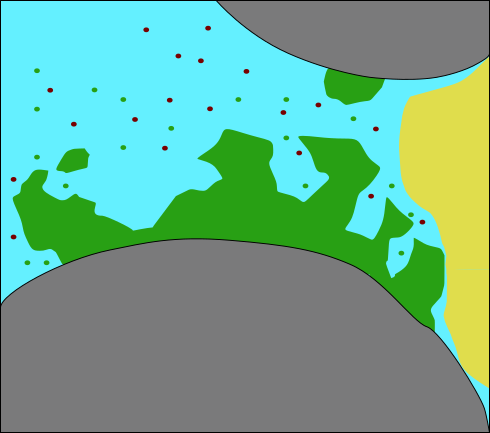 (modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
(modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
- Urea hydrolysis \begin{aligned} \underset{\text{urea}}{\mathrm{CO(NH_2)_2}} + 2 \mathrm{H_2O} \overset{\text{urease}}{\rightarrow} \\ \underset{\text{ammonia}}{\mathrm{2NH_3}} + \underset{\text{carbonic acid}}{\mathrm{H_2CO_3}} \end{aligned} ::: ::::::
MICP: Main reactions
Here: Ureolytic microbes produce the enzyme urease (MICP) \mathrm{CO(NH_2)_2 + 2 H_2O + Ca^{2+} \rightarrow 2 NH_4^+ + CaCO_3}
Different reactions in detail:
\begin{array}{lr} \mathrm{CO(NH_2)_2 + 2 H_2O \rightarrow 2 NH_3 + H_2CO_3} \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! & \text{ureolysis} \\ \mathrm{H_2CO_3 \rightleftharpoons HCO_3^- + H^+} & \text{dissociation of carbonic acid} \\ \mathrm{HCO_3^- \rightleftharpoons CO_3^{2-} + H^+} & \text{dissociation of bicarbonate ion} \\ \mathrm{2 NH_4^+ \rightleftharpoons 2 NH_3 + 2 H^+} & \text{dissociation of ammonia} \\ \mathrm{Ca^{2+} + CO_3^{2-} \rightleftharpoons CaCO_3 \downarrow} & \text{calcite precipitation/dissolution} \end{array}
Model concept: Relevant processes
:::::: {.columns}
::: {.column width=50%}
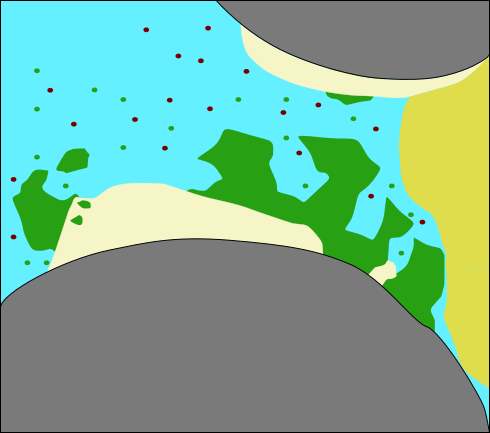 (modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=50%}
(modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=50%}
- Precipitation and dissolution of calcite
\mathrm{ \underset{\text{calcium}}{Ca^{2+}} + \underset{\text{carbonate}}{CO_3^{2-}} \rightleftharpoons \underset{\text{calcite}}{CaCO_3 \downarrow} } ::: ::::::
Model concept: Relevant processes
:::::: {.columns}
::: {.column width=45%}
 (modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
(modified after Ebigbo et al., WRR 2012)
:::
::: {.column width=55%}
-
Clogging: Reduction of porosity \phi = \phi_0 - \phi_\text{biofilm} - \phi_\text{calcite}
-
and reduction in permeability: Kozeny-Carman relation K = K_0 \left( \frac{1-\phi_0}{1-\phi} \right)^2 \left( \frac{\phi}{\phi_0} \right)^3 or the Power Law ::: ::::::
Equations
Balance Equations
:::incremental
-
Mass balance equation of components \Sigma_\alpha \frac{\partial}{\partial t}(\phi \rho_\alpha x^\kappa_\alpha S_\alpha)+ \nabla \cdot ( \rho_\alpha x^\kappa_\alpha \mathbf{v}_\alpha )- \nabla \cdot ( \rho_\alpha \mathbf{D}^\kappa_{\alpha;\text{pm}} \nabla x^\kappa_\alpha )= q^\kappa
-
Mass balance for the immobile components / solid phases: \frac{\partial}{\partial t}(\rho_\varphi \phi_\varphi) = q^\varphi
:::
Overall procedure of implementing chemical reactions in DuMu^\mathsf{x}:
- Chemical equation calculate equilibrium/kinetic reaction rate e.g. r_\text{urea}
- Reaction rate set component source/sink term e.g. q^\varphi depending on and chemical reaction
Sources & Sinks:
For this exercise:
- Neglecting microbial growth and decay, attachment and detachment as we assume a fixed biofilm for simplicity!
- We also assume that the rate of precipitation is equal to the rate of ureolysis, saving the work of detailed geochemistry calculations for the sake of both simplicity and faster run times.
Sources & Sinks:
\begin{aligned} &\text{Urea:} & q^{\text{urea}} &= -r_\text{urea} \\ &\text{Calcium:} & q^{\mathrm{Ca}^2+} &= -r_\text{precip} \\ &\text{Total carbon:}& q^{\mathrm{C}_\text{tot}^+} &= r_\text{urea} - r_\text{precip} \\ &\text{Calcite:} & q^{\mathrm{C}} &= r_\text{precip} \end{aligned}
Sources & Sinks:
\begin{aligned} \qquad\qquad & \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \text{Precipitation rate:} \\ r_\text{precip} &= f\; \left( A_\text{interface}, \Omega = \frac{\left[\mathrm{Ca}^{2+}\right]\left[\mathrm{CO_3}^{2-}\right]}{K_\text{sp}}, T \right) \\ \qquad\qquad & \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \text{For this exercise:} \\ r_\text{precip} &= r_\text{urea} \\ \qquad\qquad & \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \!\!\!\!\!\! \text{Ureolysis rate:} \\ r_\text{urea} &= k_\mathrm{urease}^\mathrm{m} k_{\mathrm{urease}, \text{biofilm}} \left(\rho_\text{biofilm} \phi_\text{biofilm}\right) \frac{m_\text{urea}}{K_\text{urea} + m_\text{urea}} \end{aligned}
Supplementary Equation:
- Updating porosity and permeability
\begin{aligned} \phi &= \phi_0 - \Sigma_\varphi \phi_\varphi \\ K &= K_0 \left(\frac{1-\phi_0}{1-\phi}\right)^2 \left(\frac{\phi}{\phi_0}\right)^3 \\ \text{or}& \\ K &= K_0 \left( \frac{\phi}{\phi_0} \right)^\eta \end{aligned}
Specific Implementations
- Update porosity in dumux/material/fluidmatrixinteractions/porosityprecipitation.hh
…
auto priVars = evalSolution(element, element.geometry(),
elemSol, scv.center());
Scalar sumPrecipitates = 0.0;
for (int solidPhaseIdx = 0; solidPhaseIdx < numSolidPhases; ++solidPhaseIdx)
sumPrecipitates += priVars[numComp + solidPhaseIdx];
using std::max;
return max(minPoro, refPoro - sumPrecipitates);
…Specific Implementations
- Update permeability in /material/fluidmatrixinteractions/permeabilitykozenycarman.hh
template<class Scalar>
PermeabilityType evaluatePermeability(PermeabilityType refPerm,
Scalar refPoro,
Scalar poro) const
{
using std::pow;
auto factor = pow((1.0 - refPoro)/(1.0 - poro), 2)
* pow(poro/refPoro, 3);
refPerm *= factor;
return refPerm;
}Biomineralization exercise
Exercise
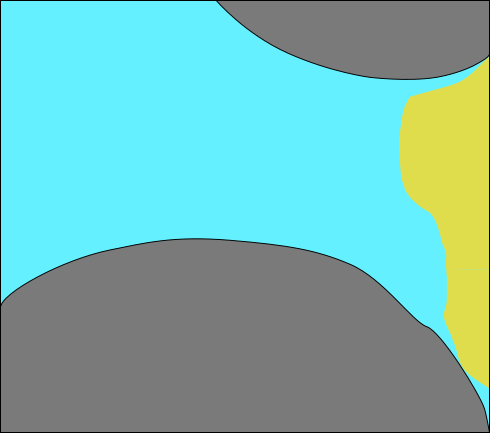
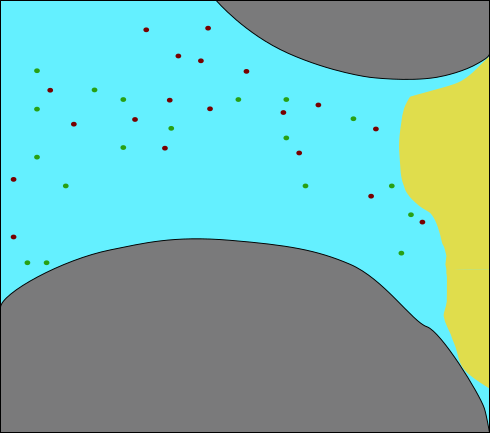
:::::: {.columns} ::: {.column width=50%}
- 2 aquifers with sealing aquitard
- Upper aquifer: "drinking water"
- Lower aquifer: "\mathrm{CO_2} storage"
- Problem:
- Leakage pathway
- Stored \mathrm{CO_2} would migrate to drinking water aquifer!
- Biomineralization to "seal" the leakage ::: ::: {.column width=50%}
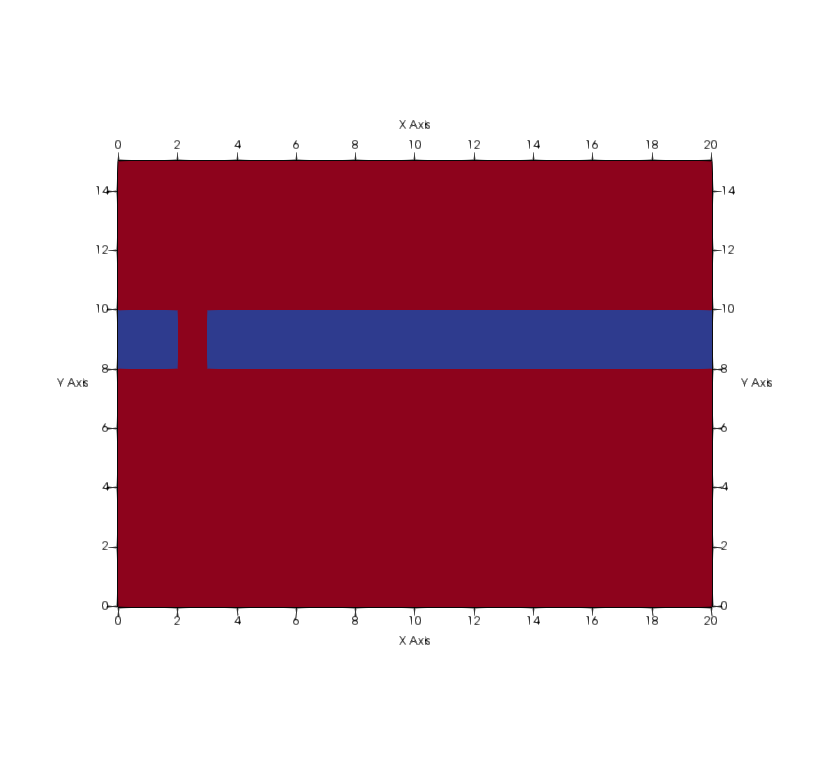 :::
::::::
:::
::::::
Exercise tasks
Exercise
First step: Go to the Biomineralization Exercise and check out the README